Carbon in the Lithosphere and Hydrosphere
The Carbon Biogeochemical Cycle in a Warming World (Article 2)
An article series by Hadi Issa
Deep in the hearts of stars, the basic chemical elements supporting life were first formed from hydrogen and helium, including carbon, nitrogen, and oxygen, contributing to the formation of celestial bodies in the solar system – the orbiting planets of Solar Nebula, including Earth. When the Earth was formed, it was covered with a hot liquid of molten minerals, known as magma. Meanwhile, magma started erupting as volcanoes, releasing gases to the atmosphere, including water vapor, nitrogen, carbon dioxide, and sulfur dioxide, forming the Earth’s atmosphere. Consequently, clouds started appearing, and heavy rain started falling, thus the Earth’s hydrosphere was formed. Afterwards, volcanoes started erupting again in the form of lava that, under cooling conditions, crystallized and formed igneous rocks, and under weathering, igneous rocks were transformed to sedimentary rocks, and later, under heat and pressure beneath the Earth’s surface, to metamorphic rocks, in a perpetual rock cycle, hence the Earth’s lithosphere was formed, and the biogeochemical cycling of chemical elements started, giving rise to life-incubating conditions.
As an essentially closed system, the Earth has a finite amount of matter. The availability of some nutrients, however, is vital for the continuity of life; therefore, those nutrients flow through continuous cycles between Earth’s subsystems, aka spheres, and from one reservoir to another, as part of the planet’s life-driving biogeochemical system. According to the law of conservation of mass – discovered by Russian scientist Mikhail Lomonosov in the mid-eighteenth century – in a closed system, matter can neither be created nor destroyed, in which both mass and energy remain constant. Mass and energy, however, undergo various physical and chemical changes that have no influence over their overall quantity in the system.
The natural processes influencing the dynamics of the carbon cycle include chemical reactions in the atmosphere, biological processes in the biosphere (respiration, photosynthesis, and decomposition), weathering and deposition of rocks along the lithosphere, and various processes in the hydrosphere (including transport, uptake, chemical reactions, and precipitation). Moreover, human activity is affecting the cycle through anthropogenic practices, mainly industrial carbon emissions that are increasing the greenhouse effect above the permissible levels, intensifying ocean acidification, and bringing up multiple pollution-related illnesses.
Article 2: Carbon in the Lithosphere and Hydrosphere
Carbon mainly reaches the Earth’s surface through rainfall events, where atmospheric carbon dioxide (CO2) reacts with the water falling during precipitation (H2O) to form carbonic acid (H2CO3), in a phenomenon known as acid rain. Carbonic acid, a weak acid, then flows through the permeable cracks and fractures on the Earth’s surface, where it chemically reacts with some kinds of natural rocks, for instance, limestone, in a process called chemical weathering, forming an aqueous solution of mineral and bicarbonate ions, as shown in the chemical equation below:
Carbonic Acid + Limestone Calcium Ions + Bicarbonate Ions
H2CO3 + CaCO3 Ca++ + 2(HCO3)-
Soluble ionic minerals are then transported to large bodies of water, including seas and oceans, through runoff, watersheds, and rivers.
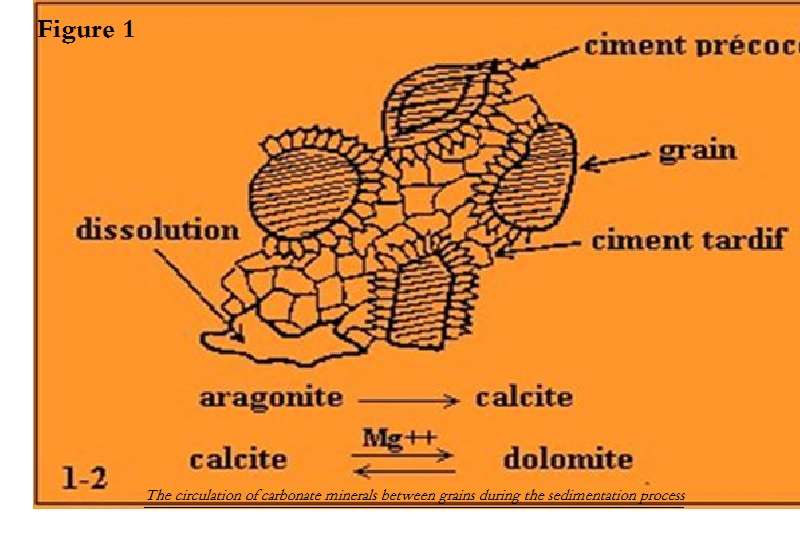
During sedimentation, the precipitation of carbonates in the pores is a rapid phenomenon and can occur in several successive phases, where the rock presents several generations of cements. It is first the compaction that intervenes and decreases the empty spaces between the elements and correlatively increases the contact areas. Meanwhile, new minerals are deposited between the grains (clay, siliceous, or carbonate minerals). Due to increase of temperature and pressure in depth, some minerals are transformed, recrystallizing according to a more regular network. Local dissolutions are possible; the observation of Coca-Cola bottles cemented in the limestone sands of the Bahamas islands is a living example of the phenomenon.
At greater depth, the crystalline species are modified: this is the level of anchizone, the beginning of metamorphism. Burial generally results in a reduction of the porosity, by bringing grain closer together and clogging the pores with cement: these porosity modifications are of great importance in the search for potential hydrocarbon reservoirs.
Figure 1: The circulation of carbonate minerals between grains during the sedimentation process
The chemical elements of sedimentary rocks come from the continental lithosphere and the atmosphere; living organisms in the biosphere can act as intermediates by concentrating or releasing certain elements (oxygen, CO2, calcium, etc.). It is essentially light elements with predominance of silicon, calcium, oxygen, carbon dioxide. Mineralogical combinations consist mainly of silicates and, incidentally, carbonates.
The ocean plays a key role in the overall carbon cycle, due to the massive amounts of carbon it uptakes through wind-driven currents, removing about 50% of the additional carbon content in the atmosphere, that it’s also known as the ‘carbon sink.’ After it has been absorbed into the ocean, carbon dioxide (CO2) chemically reacts with water molecules (H2O) to form carbonic acid (H2CO3), which is then easily broken down into hydrogen ions (H+) and bicarbonate molecules (HCO3-). Under normal conditions, when dissolved calcium ions (Ca++) are transported to the ocean through river streams, they either react with carbonate molecules (CO32-) to form calcium carbonate (CaCO3), or they get absorbed by marine shelled animals, including mussels and clams, which combine them with carbonate molecules, forming the calcium carbonate those animals need to build their shells. However, due to the presence of bicarbonate molecules, as a product of the breakdown of carbonic acid, the normal process of calcium carbonate formation is hindered from taking place, since calcium and bicarbonate don’t bind together. Under these conditions, carbonate molecules, which normally react with calcium ions, bind with hydrogen ions instead, resulting in a decrease in the PH of the seawater, in a phenomenon resulting from the presence of carbon dioxide, and consequently, carbonic acid followed by bicarbonate molecules, in the ocean, and known as ocean acidification.
Figure 2:Chemical Reactions Undergone by Ca++ Ions Under Normal Conditions (Ireland, 2009)
Figure 3: Chemical Reactions Undergone by Ca++ Ions in Case of Elevated CO2 Levels (Ireland, 2009)
Calcium carbonate is dissolved in depth; there is no more carbonate in the current seas beyond 5400 m of depth.
Carbon also exists in the ocean in the form of methane (CH4). The oxygen content of the water usually decreases with depth if the body of water is not intensely stirred; deep, calm areas are poor in oxygen, a phenomenon known as anoxia. Some bodies can only form or accumulate in anoxic environment: organic matter is fermented by microorganisms and produces methane, as well as hydrogen sulfide (H2S). The oxygen content in the ocean decreases with depth; it is zero beyond 200 m; its decrease at the surface is due to the metabolism of plankton. Hydrogen sulfide and methane, on the other hand, are produced by the bacterial decomposition of organic matter from bottom sediments.
Figure 4: Dissolved gas content in the Black Sea